What Is the Electron-pair Geometry for N in Nf3
We can also predict electron geometry via electron groups through VSEPR theory. The molecular geometry or shape of NH 3 is a Trigonal pyramidal.

1 Tetrahedral Electronic Geometry Ab 3 U Species One Lone Pair Of Electrons On A Some Examples Of Molecules With This Geometry Are Nh 3 Nf 3 Ph 3 Ppt Download
The total valence electrons available for drawing the Ammonia NH3 Lewis structure is 8.

. The four pairs of electrons arrange themselves tetrahedrally but the description of the shape only takes account of. What is the electron geometry for NF3. Molecular geometry for each molecule CF4 NF3 OF2 H2S.
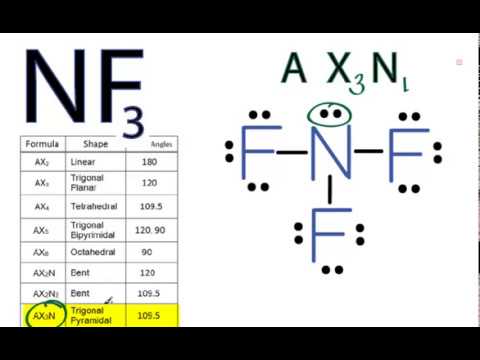
In NF3 the central nitrogen atom has four electron groups surrounding it. The general molecular geometry formula for NF3 is AX3N1. What is the hybridization of the nitrogen atom.
Electron pair geometry and molecular geometry wont be the same if there are lone pairs involved. There are lone pairs around the central atom so the geometry of NF3 is B. The shape of this molecule is trigonal pyramidal due to the presence of lone pair on the nitrogen atom.
The bond pairs arrange themselves in a trigonal planar way. According to the VSEPR chart the molecular geometry of nitrogen trifluoride is trigonal bipyramidal. In NF3 there are also three bond pairs but the nitrogen has a lone pair as well.
The bond pairs arrange themselves in a trigonal planar way. What is the electron-pair geometry for B in BH2. It is trigonal planar in shape because it only requires 3 electron pairs to fulfil its octet.
Up to 256 cash back What is the electron-pair geometry of NF. The molecular geometry of NF3 is a trigonal pyramid and its electron geometry is tetrahedral because nitrogen has Sp³ hybridization with 5 valence electrons in its valence shell and it makes three bond pairs one with each fluorine atom. Science Chemistry QA Library Draw the Lewis structure of NF3.
NF3 is trigonal pyramidal in shape. It helped me a lot. Because of these unequally shared electrons throughout the shape the molecule is polar.
What orbitals on N and F overlap to form bonds between these elements. What orbitals on N and F overlap to form bonds between these elements. The electron geometry of NH 3 is Tetrahedral.
What is the hybridization of the N atom. Up to 24 cash back But because the pair of electrons is not included in molecular geometry NF3 has a triangular pyramid shape with nitrogen at the apex. What is the electron-pair geometry for N in NFZ.
The four pairs of electrons arrange themselves tetrahedrally but the description of the shape only takes account of. Electron pairs repel each other whether or not they are in bond pairs or in lone pairs Using the VSEPR theory the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. There are lone pair s around the central atom so the geometry of NF3 is Please note that geometry refers.
What is the hybridization of the nitrogen atom. What are its electron-pair and molecular geometries. Because 1 lone pair and three bond pairs around the Nitrogen N central atom are arranged tetrahedrally.
Tetrahedral- CF4 Trigonal Pyramidal- NF3 Bent- OF2 and H2S. Nitrogen is the core atom with two electron pairs bound three N-F and one lone pair of electrons. Up to 256 cash back Draw the Lewis structure for NF 3What are its electron-pair and molecular geometries.
What orbitals on N and F overlap to form bonds between these elements. NF3 is polar in nature due to the presence of lone pair on nitrogen atom causing a distorted shape of NF3 molecule and the difference between the electronegativity of fluorine398 and nitrogen304 causes polarity in N-F bonds and result in a non zero dipole moment of the entire molecule. What is the electron-pair geometry for N in NF3.
Fluorine being more electronegative pulls the bonded electron pairs slightly more towards its side and gains a partial negative charge due to which polarity rises in the molecule. Up to 256 cash back Draw the Lewis structure for NF 3What are its electron-pair geometry and molecular geometries. Up to 24 cash back of nitrogen trifluoride.
Draw the Lewis Structure for KrF2. This is because N requires 4 electron pairs to fulfil its octet. Three single bonds three bonded pairs and one lone pair.
In NF3 there are also three bond pairs but the nitrogen has a lone pair as well. According to the VSEPR theory if the NF3 molecule ion has an AX3N1 generic formula the molecular geometry and electron geometry will both be trigonal pyramidal forms. What is the molecular geometry and polarity of NF3.
NF3 has a tetrahedral geometric structure and a trigonal pyramidal shape one nonbonding electron pair on Nitrogen.
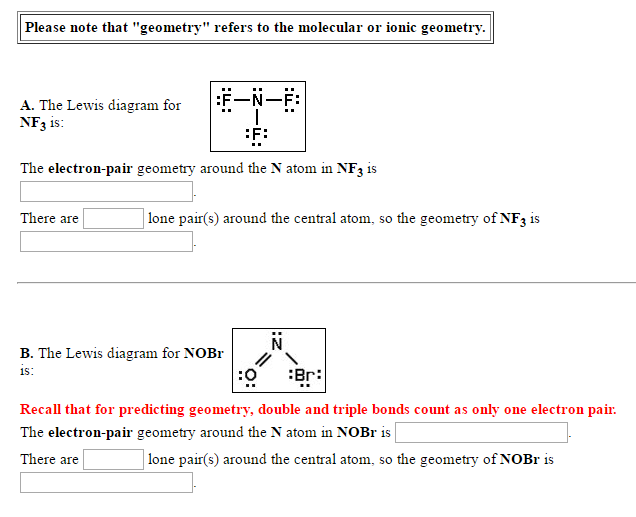
Solved That Geometty Reler Please Note That Geometry Chegg Com

Geometry Of Molecules Nf3 Lewis Structure Nitrogen Trifluoride Facebook
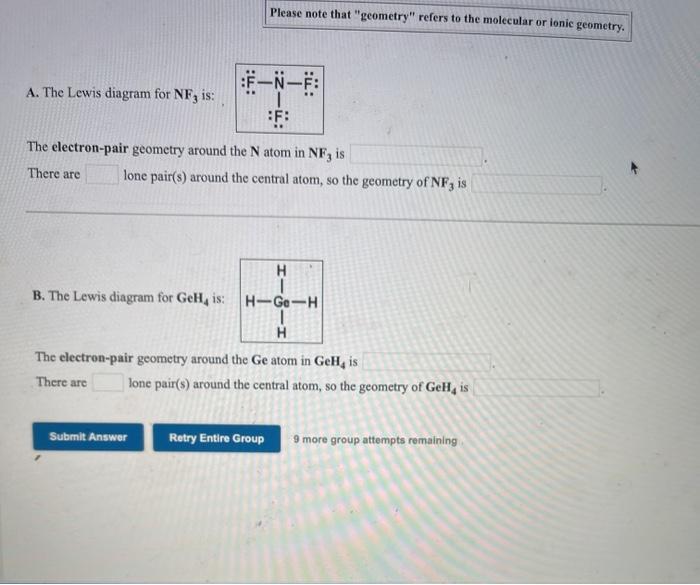
Solved Please Note That Geometry Refers To The Molecular Chegg Com

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Nf3 Molecular Geometry Science Education And Tutorials

Bf3 Molecular Geometry Science Education And Tutorials
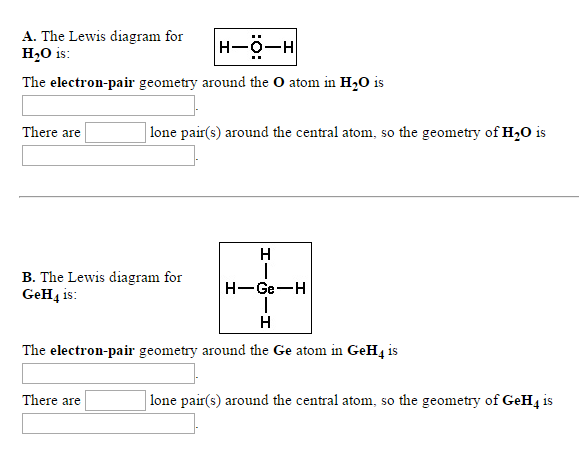
Solved That Geometty Reler Please Note That Geometry Chegg Com

Vsepr For 4 Electron Clouds Video Vsepr Khan Academy
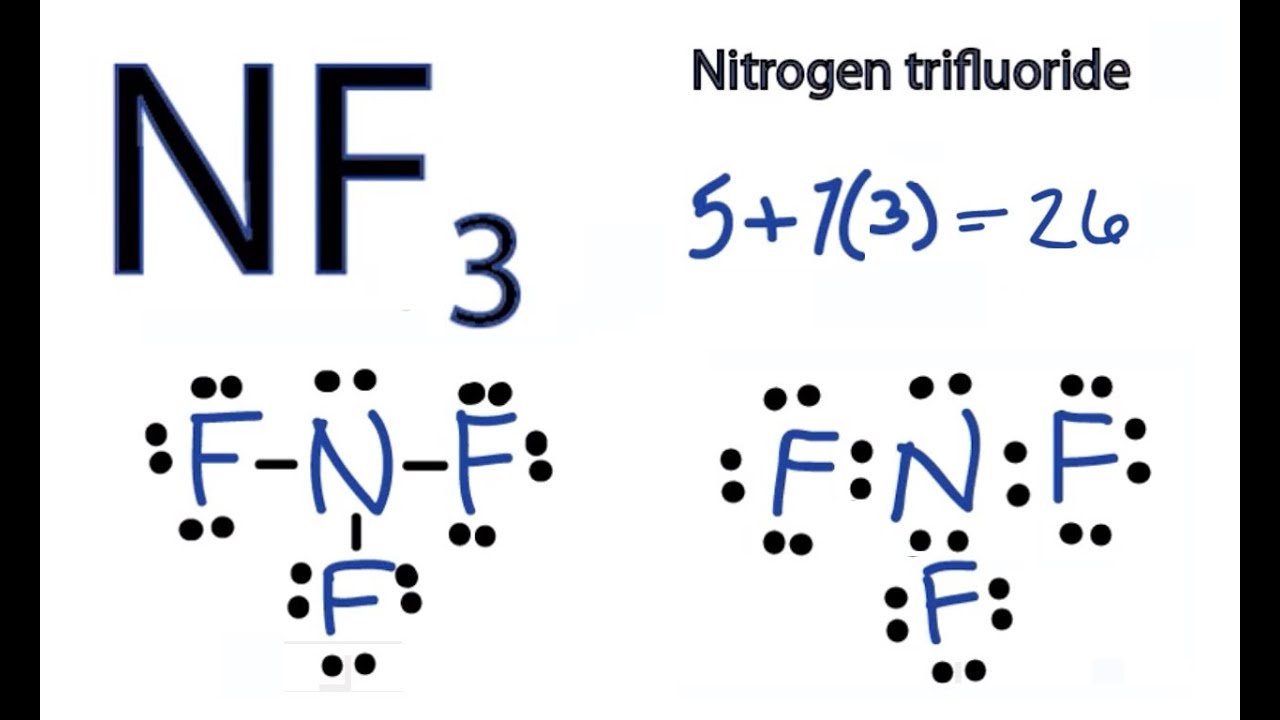
Nf3 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
Solved A What Is The Electron Pair Geometry For N In Nf3 Chegg Com

Nf3 Molecular Geometry Shape And Bond Angles Youtube

Compound Lewis Structure Total Valence Electrons Electron Pair So Molecular Geometry Approximate Polar Geometry Bond Angles Homeworklib

Nf3 Lewis Structure And Molecular Geometry Youtube

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Nf3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Nf3 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Comments
Post a Comment